Answer:
The pressure of the nitrogen in the container is 168.774 kPa
Step-by-step explanation:
The volume, pressure, temperature and mol of a gas are linked through the ideal gas law which is:
p . V = n . R . T
Where p is the pressure of the gas, V the volume of the gas, n the amount of moles of the gas, T the temperature of the gas and R the universal gas constant.
The universal gas constant R we will be using is:
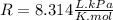
For example, What is the temperature of 4 mol of gas in a 10 Liters container at 300kPa?
The data is:
V = 10L
p = 300kPa
n = 4 moles
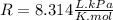
T = ?
Now we put the data in the equation and calculate the temperature.
10L ⋅ 300kPa = 4mol ⋅
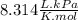 ⋅ T
⋅ T
T = 10L· 300kPa÷4mol ⋅
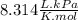
T= 90.21 K
So, knowing all the data except one, using the ideal gas law we can calculate it.
The equation for this case would be:
p = 0.5mol ·
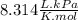 · 203K ÷ 5.0L
· 203K ÷ 5.0L
And the result:
p = 168.774 kPa