Answer: The density of copper block is, 8.92 g/mL
Explanation : Given,
Length of copper block = 5.05 cm
Breadth of copper block = 2.55 cm
Height of copper block = 1.25 cm
To calculate the volume of copper block by using the formula of volume of cuboid, we use the equation:
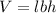
where,
V = volume
l = length
b = breadth
h = height
Putting values in above equation, we get:
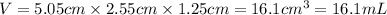
Conversion used :
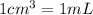
Now we have to calculate the density of copper block.
Formula used :
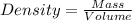
Given:
Mass of copper block = 143.584 g
Volume of copper block = 16.1 mL
Now put all the given values in this formula, we get:
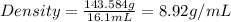
Therefore, the density of copper block is 8.92 g/mL