Answer:
234 is the mass number of the resulting element that is uranium.
Step-by-step explanation:
Beta-decay is nuclear process in which a neutron gets converted into a proton and an electron releasing a beta-particle. The beta particle released carries a charge of -1 units.
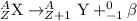
So, when protactinium-234 undergoes beta decay to form uranium-234 as resultant element.
The reaction can be expressed as:
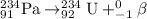
234 is the mass number of the resulting element that is uranium.