Answer:
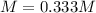
Step-by-step explanation:
Hello,
In this case, the first step is to compute the molar mass of sugar, more specifically sucrose,
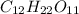 as shown below:
as shown below:
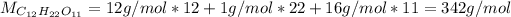
Afterwards, we compute the moles contained into 34.2 grams of sugar, based on the previously computed molar mass as follows:
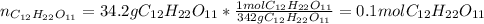
Finally, we apply the molarity equation taking into account that the 300.0 mL must be used in liters rather than milliliters, that is 0.3 L:
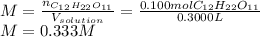
Best regards.