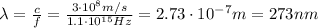1 mol of photons contained a number of photons equal to Avogadro number:
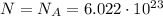
The total energy of the mole of photons is
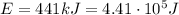
, so the energy of a single photon is the total energy divided by the number of photons:
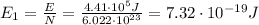
The energy of a single photon is related to its frequency f:
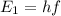
where h is the Planck constant. From this formula, we find the frequency of the photons in the problem:
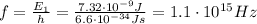
And from the frequency we can finally calculate the wavelength
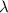
, using the relationship between wavelength, frequency and speed of light (c) for photons: