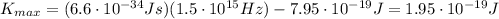In the photoelectric effect, the energy of the incoming photon (E=hf) is used in part to extract the photoelectron from the metal (work function) and the rest is converted into kinetic energy of the photoelectron:
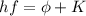
where
h is the Planck constant
f is the frequency of the incident light
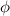
is the work function of the material
K is the kinetic energy of the photoelectron.
The photoelectron generally loses part of its kinetic energy inside the material; however, we are interested in its maximum kinetic energy, that is the one the electron has when it doesn't lose energy, so we can rewrite the previous equation as
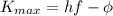
The work function is (in Joule)
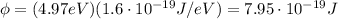
and using the data of the problem, we find the maximum kinetic energy of the photoelectrons