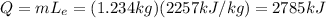During the phase transition vapour --> liquid water, the temperature of the water does not change; the molecules of water release heat and the amounf of heat released is equal to
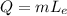
where
m is the mass of the water
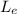
is the latent heat of evaporation.
For water, the latent heat of evaporation is
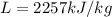
, while the mass of the water is
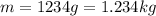
so, the amount of heat released in the process is