Answer: The mass of the ethanol is 322.6 pounds
Step-by-step explanation:
Density is defined as the mass contained per unit volume.
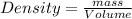
Given : Mass of ethanol = ?
Density of ethanol=
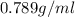
Volume of ethanol = 49.0 gal = 185485 ml ( 1gal=3785ml)
Putting in the values we get:
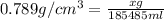
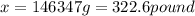 (1g=0.00220462pound)
(1g=0.00220462pound)
Thus the mass of the ethanol is 322.6 pounds