Answer is given below
Step-by-step explanation:
given data
pressure = double
volume = constant
solution
As we know that an Average velocity and rms velocity is directly proportional to square root of PV ..................1
so if we take P is doubled while keeping V constant
than Velocity increases by a factor
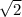
so that Factor = 1.414 for both the cases