Answer:
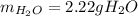
Step-by-step explanation:
Hello!
In this case, since the combustion of butane is:
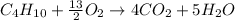
As there is an excess of oxygen, we can compute the mass of water by simply using the molar masses of butane and water and the 1:5 mole ratio between them as shown below:
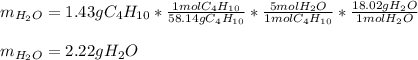
Best regards!