Answer:- The gas needs to be transferred to a container with a volume of 11.2 L.
Solution:- From Boyle's law. "At constant temperature, Volume is inversely proportional to the pressure."
It means, the volume is decreased if the pressure is increased and vice versa.
Here, the Pressure is decreasing from 537 torr to 255 torr. So, the volume must increase and calculated by using the equation:
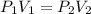
Where,
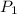 is initial pressure and
is initial pressure and
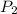 is final pressure. Similarly,
is final pressure. Similarly,
 is initial volume and
is initial volume and
 is final volume.
is final volume.
Let's plug in the values in the equation:
(537 torr)(5.30 L) = (255 torr)(
 )
)
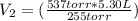
 = 11.2 L
= 11.2 L
So, the new volume of the container needs to be 11.2 L.