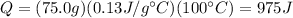The amount of heat Q needed to increase the temperature of a substance by

is given by
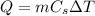
where m is the mass of the substance, and
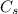
is its specific heat capacity.
In our problem, we have:
m=75.0 g
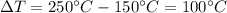
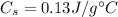
so, substituting these numbers, we get the amount of heat needed: