Answer:
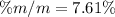
Step-by-step explanation:
Hello!
In this case, since the percentage by mass of a solute in a solvent is computed as shown below:
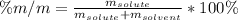
Now, as the solute here is calcium chloride and has a mass of 35.0 g and the solvent is water with a mass of 425.0 g, we can compute the percentage by mass as shown below:
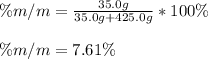
Best regards!