Answer :
(a) The name of
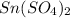 compound is, tin sulfate.
compound is, tin sulfate.
(b) The molar mass of
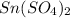 is 310.83 g/mol
is 310.83 g/mol
Explanation :
(a) As we know that the tin is the alkali metal having the atomic number 50 and belongs to group 14. The symbol of tin is, (Sn). The stable oxidation state of strontium is (+4).
The element sulfate is the non-metal polyatomic ion. The stable oxidation state of sulfate ion
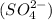 is, (-2).
is, (-2).
When the tin combine with the sulfate ion, it forms tin sulfate. It is an ionic compound in which the a metal react with the non-metal and combine with the an ionic bond by the criss-cross method.
The formula of the tin sulfate is,
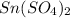
Hence, the name of
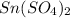 compound is, tin sulfate.
compound is, tin sulfate.
(b) Now we have to determine the molar mass of
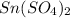 .
.
Molar mass : It is defined as the mass in grams of one mole of substance and each element has its own unique molar mass. It is expressed in grams/mole or g/mol. It is equal to the mass of atom present in 1 mole.
As we know that,
Molar mass of 'Sn' = 118.71 g
Molar mass of 'S' = 32.066 g
Molar mass of 'O' = 15.999 g
Molar mass of
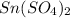 =
=
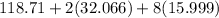
Molar mass of
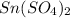 = 310.83 g
= 310.83 g
Hence, the molar mass of
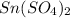 is 310.83 g/mol
is 310.83 g/mol