Answer:
(a) Group IIA.
(b) Group VIA.
(c) Group IA.
Step-by-step explanation:
Hello,
To solve this problem, one remembers that the subscripts are related with both the cation's and anion's oxidation states exchanging which are also related with the group they belong, thus:
(a) For
 since the fluoride anion has 2 as the subscript and -1 as its charge, X should belong to group IIA because X has 1 as its subscript.
since the fluoride anion has 2 as the subscript and -1 as its charge, X should belong to group IIA because X has 1 as its subscript.
(b) For
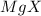 since the magnesium cation has 1 as the subscript and it belongs to group IIA, it means that it has +2 and the anion has -2 as the charges, thus, X should belong to group VIA for the nonmetals that have such oxidation state.
since the magnesium cation has 1 as the subscript and it belongs to group IIA, it means that it has +2 and the anion has -2 as the charges, thus, X should belong to group VIA for the nonmetals that have such oxidation state.
(c) For
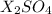 since the sulfate anion has 1 as the subscript, and X has two, X should belong to group IA.
since the sulfate anion has 1 as the subscript, and X has two, X should belong to group IA.
Best regards.