First, let's calculate how many atoms are contained in 8.00 g of gold. To do that, we should multiply the number of atoms in one gram by the mass of the gold:
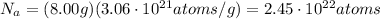
Then we know that each atom contains 79 electrons, so in order to get the total number of electrons, we should multiply the total number of atoms by the number of electrons per atom:
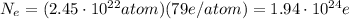
and this is the total number of electrons in 8.00 g of gold.