The atomic mass of the elements making up MgCl₂ are;
Mg - 24.3
Cl - 35.45
The formula mass of MgCl₂ is 24.3 + (2x35.45) = 95.2
One formula is made of 6.022 x 10²³ formula units of MgCl₂
the formula mass of 95.2 contains 6.022 x 10²³ formula units
Therefore a mass of 34.6 g -
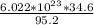
number of MgCl₂ formula units are - 2.19 x 10²³ formula units