We have that energy=specific heat * change in temperature * mass. Thus, we have the final temperature (22) minus the initial temperature (55) to equal -33 as our change in temperature. Our specific heat is in J/g*C, so we're good with that because g stands for grams and the aluminium is measured in grams. As there are 10 grams of aluminum, we have
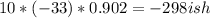
as our final temperature
An exothermic reaction would release energy and would therefore lose heat itself, while an endothermic reaction would absorb energy and gain heat. Therefore, losing heat would be an exothermic reaction
Feel free to ask further questions!