Answer:-
 atoms of Cu are equals to 4.45 moles.
atoms of Cu are equals to 4.45 moles.
Solution:- It is a atoms to moles unit conversion for which we divide the given number of atoms by Avogadro number.
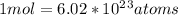
We have been given with
 atoms and asked to convert them to moles.
atoms and asked to convert them to moles.
The set up for this unit conversion problem would be as:
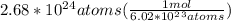
= 4.45 moles
So, there would be 4.45 moles of Cu in
 atoms of Cu.
atoms of Cu.