Answer: The correct answer is Option 1.
Step-by-step explanation:
Combustion reaction is defined as the reaction in which a hydrocarbon reacts with oxygen molecule to produce carbon dioxide and water molecule as a product.
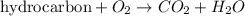
Decomposition reaction is defined as the reaction in which a large substance breaks down into two or more smaller substances.
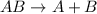
Oxidation reaction is defined as the reaction in which a substance gets oxidized by gaining oxygen or when they react with oxygen atoms.
From the given options:
Wood is totally composed of hydrocarbon and its burning requires oxygen molecule. So, burning of wood is considered as combustion reaction.
Hence, the correct answer is Option 1.