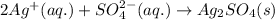Answer: The spectator ions in the solution will be
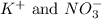
Explanation: Spectator ions are the ions which are present in the same form on both the sides of a chemical reaction.
For a given Chemical reaction:
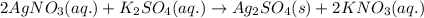
precipitate formed is silver sulfate.
The Ionic equation for the above chemical reaction is:
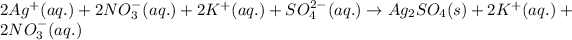
As,
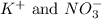 do not precipitate and hence, are considered as the spectator ions.
do not precipitate and hence, are considered as the spectator ions.
Net Ionic equation becomes: