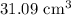Answer:
Explanation:
Given : According to legend, to determine whether the king's crown was made of pure gold, Archimedes measured the crown's volume by determining how much water it displaced.
The density of gold is 19.3 g/cm3.
The mass of the crown = 600 grams
The formula to find the density is given by :-
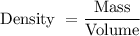
i.e.
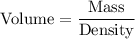
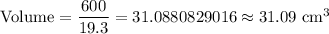
Hence, the volume of water would have been displaced if the crown was indeed made of pure gold =