Answer: 0.458 liters of HCl will be needed to neutralize LiOH.
Step-by-step explanation: Reaction follows:
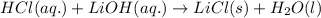
Stoichiometry ratio of LiOH and HCl is 1 : 1
which means, 1 mole of LiOH reacts with 1 mole of HCl.
Number of moles can be calculated by
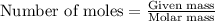
mass of LiOH = 1.65g (Given)
molar mass of LiOH = 24g
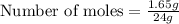
Number of moles = 0.06875 moles
Number of moles of LiOH = Number of moles of HCl
Moles of HCl = 0.06875 moles
and
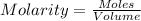
Molarity = 0.15 moles/Liter (Given)
Volume needed to neutralize LiOH will be,
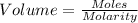
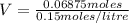
Volume of HCl needed to neutralize LiOH = 0.458 litre.