Hello!
The chemical reaction for the
neutralization of LiOH is the following:
LiOH + HCl → LiCl + H₂O
To calculate how much 0,150 M HCl would be needed, we can apply the following conversion factor, to go from grams of LiOH to mL of HCl
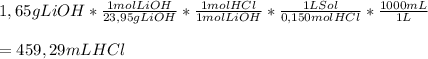
So,
459,29 mL of 0,150 M HCl are required to neutralize 1,65 g of LiOH
Have a nice day!