Hello!
This question can be easily solved using the
Mole Ratio of KNO₃/KI in the balanced reaction that is shown below:
2KI + Pb(NO₃)₂ → 2KNO₃ + PbI₂
The Mole Ratio of KNO₃/KI in this reaction is
2 moles KNO₃/ 2 moles KI so we can apply the following conversion factor:
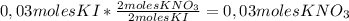
So, if 0,03 moles of KI are completely consumed,
0,03 moles of KNO₃ are formed.
Have a nice day!