Answer: The solution is saturated.
Step-by-step explanation:
We are given:
Solubility of KCl at 20°C = 34.2 g/100 g
We need to find the type of solution when 68.4 grams of KCl is added to 200 grams of water at 20°C.
There are three possibilities:
- If concentration is equal to the solubility; it is considered as saturated solution.
- If concentration is less than the solubility; it is considered as unsaturated solution.
- If concentration is greater than the solubility; it is considered as supersaturated solution.
Ratio of the solubility of two solution is:
When 34.2 gram of KCl is dissolved in 100 grams, the ratio becomes:
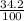
When 68.4 gram of KCl is dissolved in 200 grams, the ratio becomes:
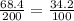
As, the ratio of the solubility of both the solutions is equal, which means that the solution is saturated.