Answer:
25 gallons of 12 % acid solution and 75 gallons of 20% solution are required.
Step-by-step explanation:
Let the volume of the solution 12% acid solution and 20% acid solution be x and y.
x + y = 100 gallons...[1]
Percentage of acid solution desired to prepare = 18%
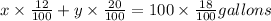 ...[2]
...[2]
On solving [1] and [2] we get:
y = 75 gallons , x = 25 gallons
25 gallons of 12 % acid solution and 75 gallons of 20% solution are required.