Answer: 0.385 moles
Explanation:-
Combustion is a type of chemical reaction in which a substance is reacted with oxygen.
The balanced chemical reaction between magnesium and oxygen is:
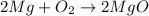
From the stoichiometry:
2 moles of magnesium react with = 1 mole of oxygen
Thus 0.770 moles of magnesium react with =
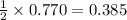 moles of oxygen
moles of oxygen
Thus 0.385 moles of
 are consumed when 0.770 mol of magnesium burns in excess oxygen.
are consumed when 0.770 mol of magnesium burns in excess oxygen.