Hello!
The precipitation reaction is the following one:
Na₂CrO₄(aq) + 2AgNO₃(aq) → Ag₂CrO₄(s) + 2NaNO₃(aq)
To know the molarity of the AgNO₃ we'll use the following molar equivalence equation:
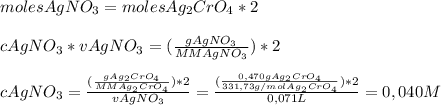
So, the concentration of AgNO₃ is
0,040 MHave a nice day!