Answer:Vrms is 149.7266 m/sec
Step-by-step explanation:The root means square velocity of a gas can be calculated as follows:
Vrms =
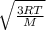
where:
R is the ideal gas constant = 8.3145
T is absolute temperature = -55 + 273 = 118 degrees kelvin
M is the molar mass of the gas = 131.293 grams = 0.131293 kg
Substituting with these givens in the above equation, we would find that the Vrms is 149.7266 m/sec
Hope this helps :)