Answer: Option (b) is the correct answer.
Step-by-step explanation:
A chemical process in which there will be absorption of heat by the reactant molecules is known as an endothermic reaction.
For example,
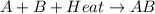
When a solid melts then actually heat energy is absorbed by it due to which bond between its molecules break. As a result, molecules start to move from their initial position as kinetic energy is gained by them.
As a result, solid state of substance changes into liquid state.
On the other hand, a chemical reaction in which there occurs release of heat is known as an exothermic reaction.
For example,
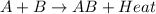
Therefore, we can conclude that the melting of a solid is an endothermic process because heat energy is absorbed.