Hello!
To solve this problem we need to know the value of
pKa for HC₂H₃O₂ (Acetic Acid) which is
4,76. Now we can apply the
Henderson-Hasselbach equation, as follows (assuming that HC₂H₃O₂ concentration is 0,22M):
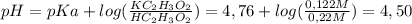
So, the pH for this buffer solution of HC₂H₃O₂ and KC₂H₃O₂ is
4,50Have a nice day!