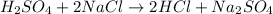Answer:- It is choice D.
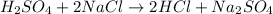
Explanations:- In general, a neutralization reaction is the reaction of an acid with base to form salt and water.
in first reaction the reaction is taking place between ammonia(a base) and hydrochloric acid(an acid) to form their salt(ammonium chloride). So, it is an acid-base neutralization reaction.
In second reaction, sodium hydroxide(a base) is reacting with acetic acid(an acid) to form their salt(sodium acetate) and water. So, it is an acid-base neutralization reaction.
In third reaction, Nitric acid is reacting with calcium hydroxide(a base) to form a salt(calcium nitrate) and water. So, it is an acid-base neutralization reaction.
In fourth reaction, sulfuruc acid is reacting a sodium chloride(a salt) to give a double replacement reaction. It is not an acid-base neutralization reaction as it's not taking place between an acid and base.
So, the correct choice is D.