Answer : The partial pressure of neon gas is, 0.61 atm
Explanation : Given,
Moles of He = 0.32 mol
Moles of Ne = 0.56 mol
Total pressure = 0.95 atm
According to the Raoult's law,
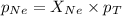
where,
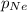 = partial pressure of neon gas
= partial pressure of neon gas
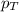 = total pressure of gas
= total pressure of gas
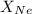 = mole fraction of neon gas
= mole fraction of neon gas
First we have to calculate the moles of fraction of neon gas.
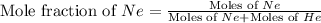
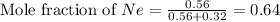
Now we have to calculate the partial pressure of neon gas.
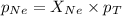
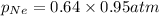
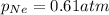
Therefore, the partial pressure of neon gas is, 0.61 atm