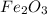Answer:
79.844 g.
Step-by-step explanation:
First we are going to calculate the molar mass of
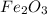
The molar mass of Fe is
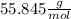 this means that 1 mol of Fe has a mass of 55.845 g.
this means that 1 mol of Fe has a mass of 55.845 g.
The molar mass of O is
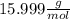 this means that 1 mol of O has a mass of 15.999 g
this means that 1 mol of O has a mass of 15.999 g
To calculate the molar mass of
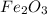 we calculate the following number :
we calculate the following number :
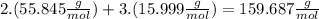
This means that 1 mol of
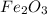 has a mass of 159.687 g.
has a mass of 159.687 g.
Finally, in 0.500 mol of
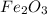 we have
we have
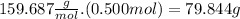
There are 79.844 g of
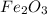 in 0.500 mol of
in 0.500 mol of