Answer:
The correct answer is option d.
Step-by-step explanation:
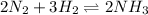
Any change in the equilibrium is studied on the basis of Le-Chatelier's principle.Whenever we increase the concentration of any species in an equilibrium ,there is a shift in an equilibrium in the direction where decrease in concentration is taking place.
So, when we add ammonia gas into the system , equilibrium will get shift in backward direction that is towards reactants.