Answer: The correct answer is Option b.
Step-by-step explanation:
Freezing point of a substance is defined as the temperature at which the solid and the liquid forms of a substance are in equilibrium. This means that the solid and liquid forms of a substance have the same vapor pressure.
It is observed that the freezing point of the solution is always lower than the pure solvent. Decreasing the vapor pressure of liquid is done by adding some solute particles to the solution. This decrease is called as depression in freezing point.
The expression for the depression in freezing point is given as:
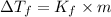
where,
 = depression in freezing point
= depression in freezing point
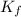 = Cryoscopic constant
= Cryoscopic constant
m = molality of the solution
Hence, the correct answer is Option b.