Hello!
The chemical reaction for the dissociation of Acetic Acid is the following:
CH₃COOH + H₂O ⇄ CH₃COO⁻ + H₃O⁺
To solve the problem we are going to use the Henderson-Hasselbach Equation, as follows, but first we need to know the pKa:
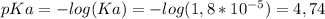
![pH=pKa + log( ([CH_3COO^(-) ])/([CH_3COOH]) )](https://img.qammunity.org/2019/formulas/chemistry/college/jiid8gfe72mhhj5407eqwns82zd4dlmwzg.png)
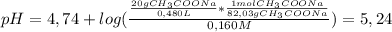
So, the pH of this solution is
5,24Have a nice day!