Charles law gives the relationship between volume and temperature.
It states that at constant pressure, volume of gas is directly proportional to temperature of gas.
V/T = k
where k - constant
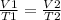
where parameters for the first instance are on the left side and parameters for the second instance are on the right side of the equation
temperature should be given in K
T1 - 90.4 °C + 273 = 363.4 K
T2 - 0.5 °C + 273 = 273.5 K
Substituting values in the equation
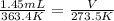
V = 1.09 mL
Final volume is 1.09 mL