The maximum kinetic energy of the electrons will be the same as the original.
In fact, in the photoelectric effect one photon hits the surface of the metal and gives its energy to one photoelectron, according to the equation:
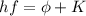
where
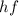
is the photon energy, with h being the Planck constant and f the frequency of the light,
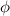
is the work function (the energy needed to extract the photoelectron from the metal) and
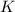
is the kinetic energy of the photoelectron.
So the kinetic energy of the photoelectron is
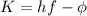
and we can see that this value doesn't change when we change the intensity of the light: in fact, if the intensity of the light is double, then the energy of the single photon hf remains the same (we have only doubled the number of photons, but not the energy of the single photon), so the kinetic energy of the electrons doesn't change.