Answer : The voltage of galvanic cell made with zinc and aluminum is, 0.90V
Explanation :
We are taking the value of standard reduction potential form the standard table.
![E^0_([Al^(3+)/Al])=-1.66V](https://img.qammunity.org/2019/formulas/chemistry/high-school/p3u0mvl80tf0v9unpwm2xkyf54buoq5qfe.png)
![E^0_([Zn^(2+)/Zn])=-0.76V](https://img.qammunity.org/2019/formulas/chemistry/high-school/7ssz7t3n2dzfcir276onkabeyvkn38kdhs.png)
In this cell, the component that has lower standard reduction potential gets oxidized and that is added to the anode compartment. The second forms the cathode compartment.
The half oxidation-reduction reaction will be :
Oxidation :

![E^0_([Al/Al^(3+)])=1.66V](https://img.qammunity.org/2019/formulas/chemistry/high-school/6qv98v04iaeshke8kqlydisc5tw8fhj1p8.png)
Reduction :
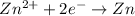
![E^0_([Zn^(2+)/Zn])=-0.76V](https://img.qammunity.org/2019/formulas/chemistry/high-school/7ssz7t3n2dzfcir276onkabeyvkn38kdhs.png)
The expression for standard cell is,
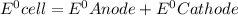
![E^0=E^0_([Al/Al^(3+))+E^0_([Zn^(2+)/Zn])](https://img.qammunity.org/2019/formulas/chemistry/high-school/yb1b52kulub5a4mf48p097glqjmf85ajb6.png)

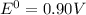
Therefore, the voltage of galvanic cell made with zinc and aluminum is, 0.90 V