Answer:Boiling point point of water at 0.54 atm is 201.42 K
Step-by-step explanation:
Boiling point of water at 1 atm = 373 K
Boiling point of water at 0.53 atm =
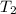
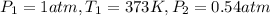
According Gay lussac's law:
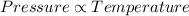 (at constant volume)
(at constant volume)
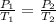
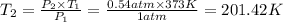
Boiling point point of water at 0.54 atm is 201.42 K