Answer:
YES
Step-by-step explanation:
Titration is an analytical method or technique which is used to determine the concentration of an unknown solution, called analyte; by using a solution of known volume and concentration, called titrant.
In this method, the volume of the titrant used to neutralize the given analyte is noted and then the concentration of the unknown solution or the analyte is calculated by using the following equation:
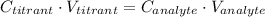
Here,
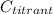 is the known concentration of the titrant
is the known concentration of the titrant
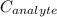 is the unknown concentration of the analyte
is the unknown concentration of the analyte
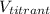 is the volume of the titrant used during the titration
is the volume of the titrant used during the titration
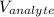 is the known volume of the analyte
is the known volume of the analyte
Therefore, in order to determine the concentration of the unknown solution or the analyte, the exact volume taken should be known.