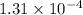Answer: The solubility product for the given salt is
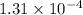
Step-by-step explanation:
Solubility product is defined as the product of concentration of ions present in a solution each raised to the power its stoichiometric ratio. It is represented as
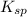
Silver carbonate is an ionic compound formed by the combination of 2 silver ions and 1 carbonate ions.
The equilibrium reaction for the ionization of silver carbonate follows the equation:
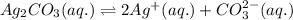
1 mole of silver carbonate produces 2 moles of silver ions and 1 mole of carbonate ion
The expression of
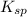 for above equation is:
for above equation is:
![K_(sp)=[2Ag^+]^2[CO_3^(2-)]](https://img.qammunity.org/2019/formulas/chemistry/college/sees8r6wbn7oyggxuvtghgbcvl4ild69n6.png)
We are given:
![[Ag^+]=(2* 0.032)=0.064M](https://img.qammunity.org/2019/formulas/chemistry/college/8ica1rqdgq0kjjcz3wfscghele6opytdha.png)
![[CO_3^(2-)]=0.032M](https://img.qammunity.org/2019/formulas/chemistry/college/k1k5jtwu7su5sm1eubza7kh74cyqyabdsg.png)
Putting values in above equation, we get:
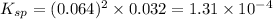
Hence, the solubility product for the given salt is