Answer: sodium chloride
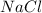 and sulphuric acid
and sulphuric acid
 are reactants .
are reactants .
sodium sulphate
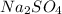 and hydrochloric acid
and hydrochloric acid
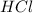 are products.
are products.
Step-by-step explanation:
Reactants of a chemical reaction is defined as the substances which take part in a chemical reaction and undergoes a chemical change. Reactants are written on left side of the arrow.
Products are defined as the substance which are formed at the end of the chemical reaction.Products are written on the right side of the arrow.
Double displacement reaction is one in which exchange of ions take place.
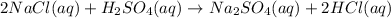
Thus sodium chloride
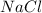 and sulphuric acid
and sulphuric acid
 are reactants and sodium sulphate
are reactants and sodium sulphate
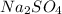 and hydrochloric acid
and hydrochloric acid
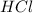 are products and all are present in aqueous (aq) state.
are products and all are present in aqueous (aq) state.