Answer : The number of moles of carbon are, 36.4 moles.
Explanation : Given,
Moles of
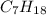 = 5.2 mole
= 5.2 mole
First we have to determine number of carbon and hydrogen atoms present in
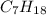 .
.
As we know that, in
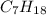 there are 7 atoms of carbon and 18 atoms of hydrogen.
there are 7 atoms of carbon and 18 atoms of hydrogen.
Now we have to determine the moles of carbon.
As, 1 mole of
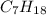 contains 7 moles of carbon.
contains 7 moles of carbon.
So, 5.2 moles of
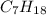 contains
contains
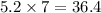 moles of carbon.
moles of carbon.
Hence, the number of moles of carbon are, 36.4 moles.