Answer: The net ionic equation is written below.
Step-by-step explanation:
Net ionic equation of any reaction does not include any spectator ions.
Spectator ions are defined as the ions which does not get involved in a chemical equation. They are found on both the sides of the chemical reaction when it is present in ionic form.
The chemical equation for the reaction of silver nitrate and sodium bromide is given as:
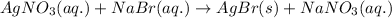
Ionic form of the above equation follows:
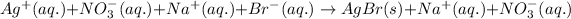
As, sodium and nitrate ions are present on both the sides of the reaction. Thus, it will not be present in the net ionic equation and are spectator ions.
The net ionic equation for the above reaction follows:
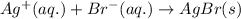
Hence, the net ionic equation is written above.