To balance the equation, Hydrogen ion should be added to the left of the equation and water to the right of the equation as follows:
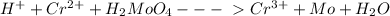
Balancing the equation
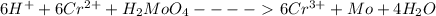
It can be noted that: ions are balanced at 18+ on left hand side and 18+ on right and side just like all the other chemical elements.