Answer:
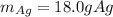
Step-by-step explanation:
Hello,
In this case, the undergoing chemical reaction is:
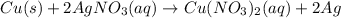
Now, since the theoretical yield of silver is required, we first must identify the limiting reactant by comparing the available moles of copper and the moles of copper that are consumed by the 28.4 g of silver nitrate as shown below:
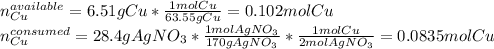
Hence, since there are more available moles of copper than consumed, we understand copper as the excess reactant and silver nitrate as the limiting one, therefore, the theoretical yield of silver turns out:
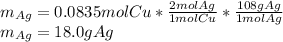
Best regards.