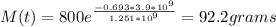Ans: Amount of K-40 remaining = 92.2 g
Given:
Initial mass of potassium-40 = M₀ = 800 g
Time, t = 3.9 *10⁹ years
To determine:
The mass of potassium-40 (M(t)) that will remain after time 't'
Step-by-step explanation:
The radioactive decay equation is given as:
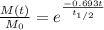 ----(1)
----(1)
where t1/2 is the half-life of K-40 = 1.251*10⁹ years
Substitute for M₀, t and t1/2 in equation(1):